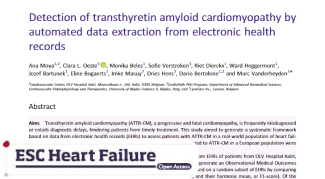
Unlocking Insights
in aTTR-CM
LynxCare's comprehensive data network offers fresh insights into patients with aTTR-CM, including but not limited to comorbidity factors, treatment patterns, and adverse events. With 400+ estimated patients and 167 variables measured, our granular and quality-controlled real-world dataset can unlock invaluable insights into the treatment and prognosis of aTTR-CM empowering clinicians to make informed decisions. Equally, we can support in identifying patients-at-risk of aTTR-CM.
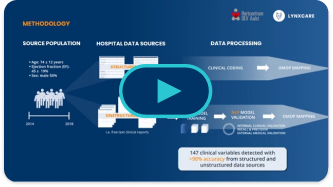
Closing the Gap
Gaps in the diagnosis and treatment of aTTR-CM in the clinical setting
Diagnostic challenge
As there is no specific ICD-10 marker code for aTTR-CM, diagnosis requires both amyloidosis (identified by E85. ICD-10 code or a tafamidis meglumine delivery) and a cardiovascular condition (identified by ICD-10 or medical procedure codes related to either heart failure, arrhythmias, conduction disorders or cardiomyopathies), which are not necessarily reported at the same visit.
New insights
The prevalence data of aTTR-CM is limited and not well characterized, due to missed and delayed diagnosis in most patients, its heterogenous clinical presentation, and previously lacking sensitive diagnostic modality.
The emergence of effective therapies for patients with amyloid transthyretin (ATTR) amyloidosis, and specifically cardiac ATTR amyloidosis, is a relatively recent and exciting development.
Advancing Research in aTTR-CM
Peer-reviewed Publication and Presented Conference Abstracts
Unveil new and deeper insights
Fostering research and collaboration
Through multi-use datasets concerning aTTR-CM patients - Heart Failure, we aim to analyze
- Difference in demographics & patient characteristics at diagnosis
- Description of diagnostic delay and patient journey from onset till diagnosis, including diagnosed with and without treatment
- Patient journey & outcome after start of treatment (Heart Failure related readmission, overall survival)
- Treatment patterns in the real world – concomitant drugs + morbidities
- Health resource utilization
Reach out if you wish to discuss and gain further insights into our research protocol and objectives.
World-Class Expertise
The LynxCare Advantage
Representativity
We strive for comprehensive representativity by including data from a mix of academic and non-academic centers. This approach allows to capture the real-world patient and treatment pathway.
Data Experts
Our team of data experts has a combined 50+ years of experience in healthcare data analytics and quality control.
Clinical Experts
We partner with clinical experts in aTTR-CM and other specialties to ensure our data offers relevant and valuable insights for healthcare professionals.
Hospital Network & Data Access
Hospitals remain in control of their own data. Each RWE request is individually handled following data governance guidelines and timelines as agreed in our Master Service Agreement to accelerate the delivery of clinical insights.
Partnership with the best statistical firms
LynxCare partners with statistical firms who already have a working relationship with life science companies.
Differentiating factors from other NLP providers & established RWD vendors
Differentiating Factors
Unlock Insights
How Hospitals Can Benefit from a LynxCare Database
Multi-purpose use of a LynxCare OMOP-CDM NLP-enriched Hospital Database
Improving Patient Care
-
Find more relevant medical information thanks to LynxCare's disease-specific NLP pipeline for a better patient and population understanding
Research & Network Opportunities
- Initiate or participate in national and international multi-center, multi-country RWE studies
- Benefit from network of KOLs
Scientific Research
- Initiate or participate in (pharma- or physician initiated) peer-reviewed (multi-center, multi-country) publications
Increased Clinical Trial Participation
-
More effective patients/cohort selection through clinical NLP, enrolling more patients in clinical trials
Improve your Data Quality
- Receive feedback on the clinical data quality of your hospital’s database
- Benefit from data science training provided

The Future of aTTR-CM Treatment
How LynxCare Can Help
Personalized Medicine
By providing insights into individual pathways of aTTR-CM patients, our data asset enables clinicians to tailor treatments to specific patient needs, opening up the possibility of personalized medicine.
Improved Outcomes
By providing insights into the clinical pathway of patients diagnosed with aTTR-CM, our data asset aims to ultimately improve patient outcomes by providing valuable insights for clinicians and researchers.
Continued Innovation
With our comprehensive dataset and world-class expertise, we aim to continue to drive innovation in the treatment and management of aTTR-CM patients, ensuring that the latest insights and solutions are available to healthcare providers and researchers.
* LynxCare datasets concern datasets within the LynxCare's European hospital network. The hospitals are the data controllers. No commitment before site approval on RWE request.